|
Consulting and Development——Delivering exciting solutions to challenging clients. |
||
|
|
||
 |
||
|
SENMAY boasts an expert team in research and development, industrialization, molding, manual and automated assembly, quality, and regulatory support, making us the ideal partner for customized diagnostic consumable solutions. We provide assistance throughout the entire product lifecycle, from conceptual development, product design, process optimization to industrialization. We support you from the conceptual stage, leveraging the expertise of our developers and engineers. Our goal is to create a technologically mature component tailored specifically to your application and capable of stable serial production. This may involve adjustments to geometry and tolerances, or even changes to planned plastic materials. Our modular CDMO approach for medical diagnostics and laboratory products allows flexible entry into various stages based on your project status. We collaborate on plastic system solutions, design process-optimized injection molds, assess economic viability, calculate variants for batch production, and establish quality control objectives. Considerations include production and post-processing (such as marking, assembly of multiple components, sterilization methods), as well as application-related requirements (such as temperature resistance). At this stage, the quality department provides support for quality planning, particularly in areas such as blueprint design, measurement strategy for series production, and design FMEA. |
|
* Product design
* Product development
|
|
Ultra-Precision Mold Making——High-quality tooling for manufacturing long-lasting, accurate parts. |
||||
|
|
||||
|
Our clients benefit from our in-house mold manufacturing, which emphasizes quality and performance. A qualified and proactive technical team manufactures single-component and dual-component ultra-precision molds. In addition to standard techniques, we utilize precision CNC machining centers to manufacture intricate structures and employ laser technology to produce components layer by layer. Highly automated and standardized production takes place on state-of-the-art equipment. System-integrated measurement technology verifies each part on every tool, ensuring seamless quality assurance. With all these factors working together, our tool manufacturing is regarded as the precision core. |
||||
 |
||||
|
* Ultra-precision molds with single-layer, multi-layer, and other multi-cavity configurations.
|
||||
|
OEM production and processing of medical components——production process in all qualification and validation stages |
|
|
|
|
|
We provide highly specialized system solutions for clients in pharmaceuticals, medical, dental, surgical technologies, biotechnology, microfluidics, and diagnostic fields. All production processes adhere to medical technology standards, complying with qualification certifications and validations at all stages. Our cleanroom production at ISO 7 and 8 levels adheres to DIN EN ISO 14644 standards, ensuring maximum safety and hygiene conditions. We have obtained various certifications, including ISO 13485 (Medical Device Quality Management System), to deliver medical-related products. Additionally, we can manufacture and assemble products in cleanrooms. Moreover, our 24/7 automated production lines enable faster and more cost-effective product delivery.
|
|
|
|
|
|
* We have over 230 high-precision injection molding machines in our factories in Shenzhen and Dongguan, along with a controlled cleanroom area of 7,600 square meters (GMP, ISO 8, and ISO 7 levels).
|
|
|
Medical component assembly and finishing——customer-focused and in compliance with the strictest hygiene standards |
|
|
|
The final assembly, labeling, and packaging of medical components are conducted in ISO 7 and ISO 8 cleanroom conditions according to DIN EN ISO 14644 standards to meet the stringent requirements of customers in the medical technology sector. Modular assembly equipment provides flexible and expandable solutions. By employing modularization, we can offer tailor-made solutions that meet high-quality standards even when scaling up production. Modularization enhances production efficiency, reduces costs, shortens time to market, and enables increased capacity.
|
|
|
|
Contract development and manufacturing (OEM) of precision medical consumable products |
|
CELL CULTURE MICROPLATES |
STORAGE PLATES |
 |
 |
|
* Manufactured according to DIN ISO 9001 standards, traceable back to production through a defined batch numbering system.
|
* Made from high-purity polypropylene material (48/96/384/1536 wells), with excellent chemical compatibility. Transparent walls allow easy visualization of contents.
|
|
PIPETTE TIPS |
BLOOD COLLECTION TUBES |
 |
 |
|
All pipette tips, regardless of type, surface, or shape, adhere to the same high standards of quality. We ensure that our products are manufactured and tested according to international standards and meet the required specifications. They are free from detectable DNase, RNase, human DNA, PCR inhibitors, and endotoxins (pyrogens). Additionally, they are non-cytotoxic and tested according to ISO 8655-6. Flexibility is crucial for modern liquid handling solutions. To ensure optimal functionality and fit, our pipette tips are made of high-quality polypropylene. |
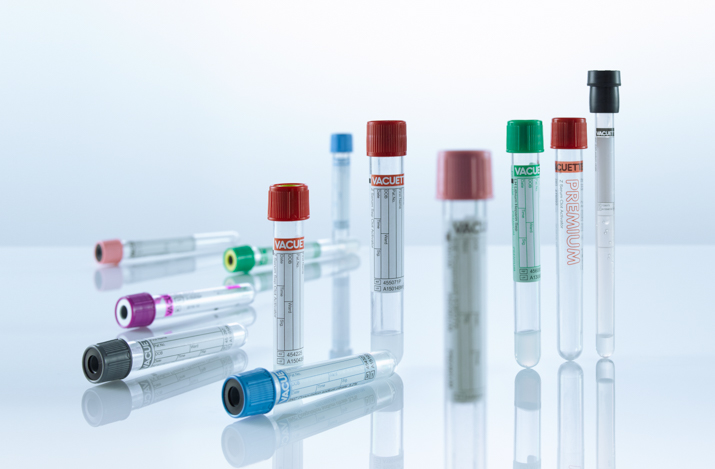
SENMAY offers a variety of vacuum blood collection tubes for coagulation tests, serological tests, EDTA hematological tests, and various specialized tests. Almost all blood collection tubes contain chemical additives and have strictly regulated negative pressure to maintain the correct mixing ratio between the additive and the extracted blood. Easy to use and providing highly reliable test results, they have become the "original tubes" for commonly used analytical devices. All blood collection tubes are CE marked.
|
|
MEDICAL SYRINGE |
CELL STRAINER |
 |
 |
|
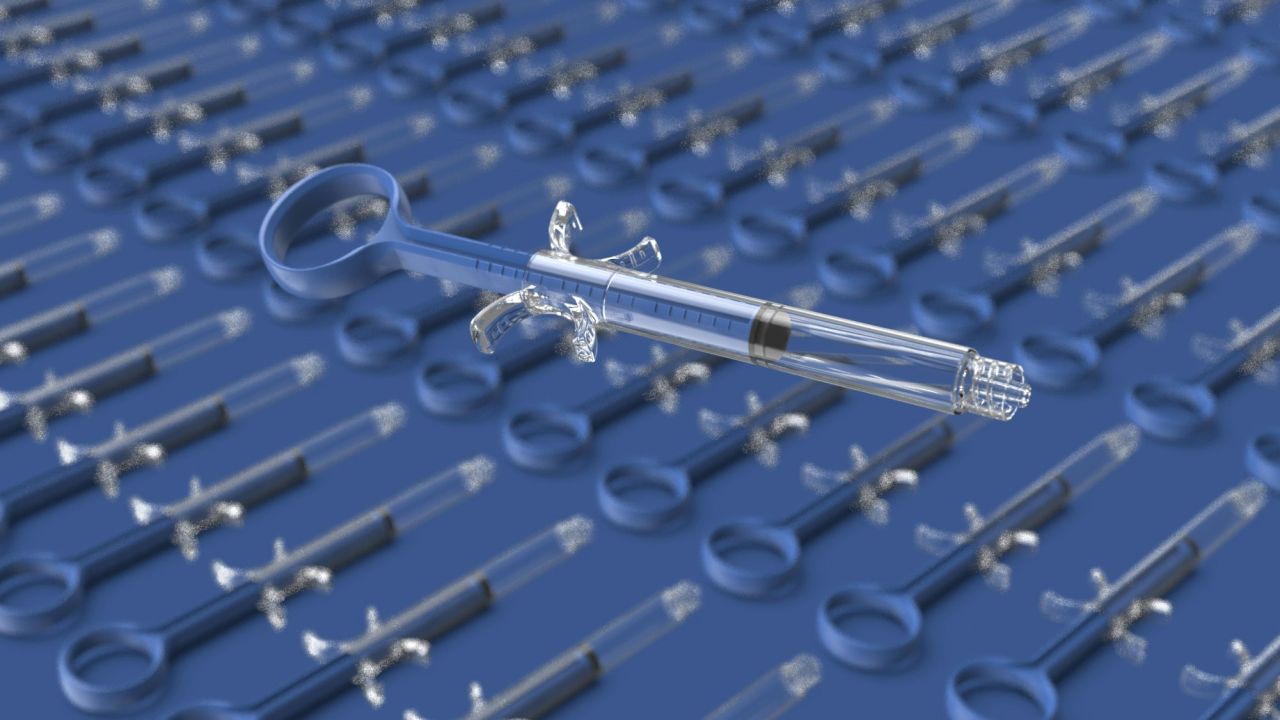
* The manually retractable safety syringe is designed to prevent needlestick injuries and reuse of the syringe by retracting the needle into the barrel. * Compatible with all standard subcutaneous injection needles. * Easy to use. * During activation of the safety mechanism, fingers remain behind the needle. * Clear, bold scale markings. * Visual and auditory confirmation of safety mechanism activation. * The barrel is made of polypropylene, and the plunger is made of polyethylene. * Free from DEHP, PVC, and not made from natural rubber latex. * Sterile and for single-use only. * Sterilization method: Ethylene oxide. * CE marking. |
* Available in 3 sieve pore sizes: 40 µm, 70 µm, and 100 µm. * Offered in 3 different colors: blue, white, and yellow for easy identification. * Enhances uniformity of single-cell suspensions. * Constructed from durable nylon mesh with uniform mesh spacing. * Extended lip on the filter allows for aseptic manipulation using forceps. * Molded color-coded polypropylene frame with labeled tags for easy handling and identification. * Designed to fit perfectly with 50 mL conical tubes. * Ready-to-use, gamma-irradiated for sterilization, non-cytotoxic. * Individually packaged, disposable, easy to use, cost-effective, maintains sample integrity. * Free from thermal sources. |
|
PCR TUBES AND PLATES |
Inoculating Loops&Needle |
 |
 |
|
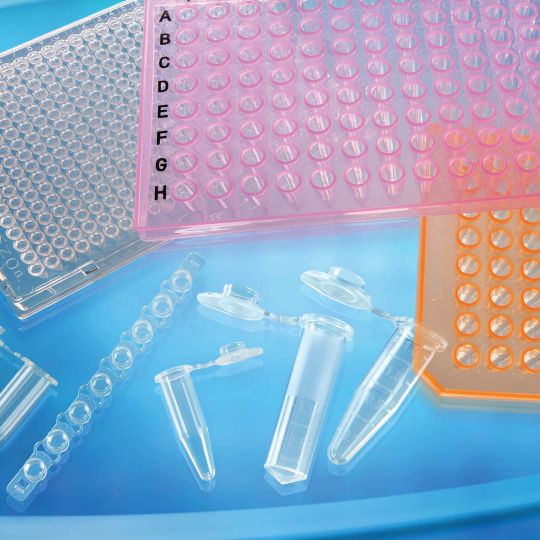
Deep well microplates are designed for absorbance, fluorescence, or luminescence assays, offering different bottom configurations to meet various needs. PCR tubes are made of high-quality polypropylene with uniform thin walls for efficient heat transfer. The lids are compatible with thermal cyclers, allowing high-pressure sterilization to ensure sealing and prevent sample evaporation.Compliant with ANSI/SBS standards, compatible with most thermal cyclers and sequencers. * Two plate types available: 96-well and 384-well, available as skirted or semi-skirted plates depending on user requirements. * Available in two versions: 0.2ml single tubes and 0.2ml 8-tube strips, suitable for general PCR experiments. * Excellent airtightness, easy to open without contamination. * Flat lid design with good flexibility and strong corrosion resistance. * PCR plates, skirtless flat plates, and skirtless raised well plates available. * Free from DNAse/RNase contamination, human genes, and endotoxins. * Customization services available. |
Inoculation loops and needles are designed for quantitative procedures such as sampling, serial dilution, and bacterial inoculation. They are particularly suitable for anaerobic microbiological applications that require deep agar inoculation. They are smooth and flexible, aiding in even, smooth streaking without damaging the gel surface. The needles are straight, ideal for removing single colony specimens. Disposable inoculation loops and needles do not require flaming, eliminating the risk of pathogenic aerosol formation and infection. They eliminate cross-contamination caused by improper sterilization. Sterile packaging is provided in secure, tamper-evident, and resealable zip-lock bags. * Precision molding * Non-toxic * Gamma radiation sterilization * Color coding for product identification * Available in 1μL and 10μL inoculation volumes * Made of polystyrene (PS) and high-impact polystyrene (HIPS) |
|
Microfluidic Plate |
Needleless syringe |
 |
 |
|
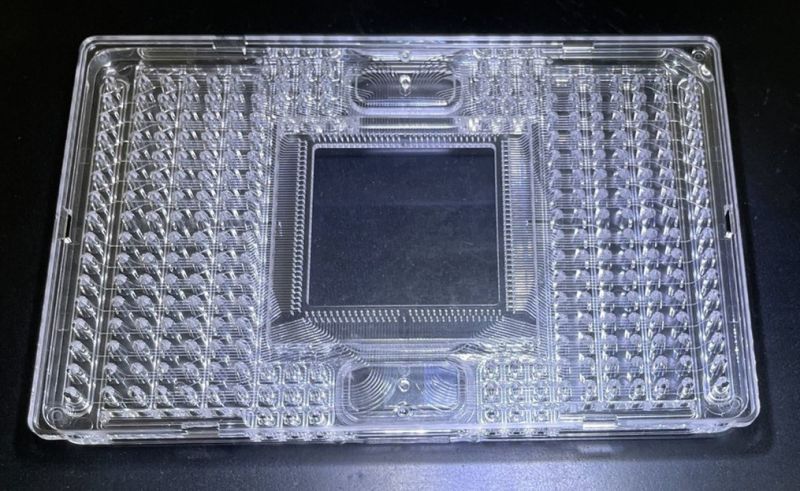
In the microfluidic industry, the most complex product consists of 260 channels 0.1 mm wide and 520 holes 0.4 mm in diameter on a ISO-standard 127.6mm x 85.48mm tray, and to meet optical characteristics, no mark is allowed by microscopic inspection at 200 times magnification. In order to realize this extremely difficult product shape, it is essential to have mirror-finishing mold surface polishing technology even for uneven shapes, micron-order machining accuracy, and skilled assembly and finishing skills. We are proud of our many achievements as professionals who possess all of these skills. |
From a young age, we probably experience injections in many aspects of our lives, such as vaccinations, dental treatment, insulin administration for diabetes, and intravenous drips. If there were a syringe that was easy to use, safe, and less painful, surely it would enable safer treatment for both patients and medical professionals. The "needle-less syringe" uses unique technology to reduce the pain of needles by using a fine jet stream rather than inserting a needle. |
|
Quality management——maximum safety and transparency – the basis for excellence and precision |
|
|
|
|
 |
|
|
We rely on systematic, process-oriented, and proactive quality assurance. To achieve this goal, we continuously invest in the technical expertise and professional knowledge of our employees. They also provide necessary documentation to ensure maximum transparency. |
* Management systems comply with ISO 9001, ISO 13485, and ISO 14001. |





